Is it possible to develop and optimise sorbent materials for sustainable ammonia synthesis?
May 17, 2023
Green ammonia (NH3) is an essential compound for the decarbonisation of the energy system of the future. It is a prominent hydrogen storage molecule (hydrogen density of 10.7 kgH2/100L) and it can be liquefied at 1 MPa and 25°C, making easy its transportation. However, ammonia production suffers from thermodynamic constraints. High pressures (>100bar) and high temperatures (>400°C) are typically required in the industrial Haber-Bosh (HB) process, resulting in significant energy consumption and reactor volumes.
With the introduction of ammonia sorbent materials in the reactor, it is possible to shift the equilibrium towards the product (NH3), increasing the hydrogen conversion. The use of sorbent materials has the potential to reduce the pressure and the temperature needed for the ammonia synthesis process, making it more efficient and less energy intensive.
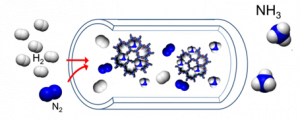
In the framework of the HySTrAm project, Eindhoven University of Technology aims to synthetise, characterise and test materials capable of effectively absorbing ammonia allowing the integration of ammonia synthesis and absorption. The university will experimentally investigate the sorbents multicyclic stability at milder conditions than the conventional HB process (i.e T<400˚C and p20 bar). The results of these studies will allow us to select the best preforming material aiming at the highest ammonia sorption capacity.
During the first year of the project, Eindhoven University of Technology has developed a first batch of sorbent materials at lab scale following scalable synthesis procedures and varying different parameters such as active phase and support.
To understand the influence of the tuning parameters listed above, these materials are firstly characterised using X-ray photoelectron spectroscopy (XPS) analysis, to determine the chemical composition of the surface, X-ray diffraction (XRD) analysis, to determine the crystalline phase, Brunauer-Emmett-Teller (BET) and Barrett-Joyner-Halenda (BJH) elaboration of the N2 adsorption–desorption isotherms, to determine the specific surface area (S.A.) and pore volume (P.V.) and finally the Inductively Coupled Plasma Atomic Emission Spectroscopy (ICP-AES) to evaluate the bulk chemical composition.
For some of those materials, they have already tested the sorption capacity and their multicyclic stability in a small quartz tube reactor (schematic reported in Figure below) under exposure to a mixture of NH3 and N2 in a range of 150-300˚C and up to 8 bar.
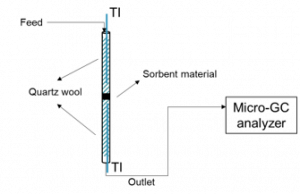
Schematic of the ammonia sorption tests setup.
During the upcoming months, the university will identify the best performing sorbent for ammonia by testing sorption capacity and multicyclic stability in a new experimental rig where it is possible to operate at conditions relevant for the HYSTRAM project, i.e, temperatures up to 300 ˚°C and pressures up to 40bar.
 SHARE ON
SHARE ON